
Rutherford Scattering Experiment Diagram, Observations and Results
Rutherford Scattering Experiment is a classic physics experiment that led to the discovery of the atomic nucleus. By observing how alpha particles scatter after striking a thin gold foil, Rutherford showed that atoms have a small, dense center, overturning earlier models of atomic structure crucial for JEE Main Physics understanding.
This experiment, also called the Gold Foil Experiment, is not only foundational in historical physics but also directly relevant to numericals and conceptual questions in JEE Main. Mastering the experimental method, key observations, and its formulas such as the Rutherford scattering cross-section is essential preparation for the exam.
Introduction and Definition: Rutherford Scattering Experiment
The Rutherford Scattering Experiment was designed to investigate the internal structure of the atom. In simple terms, it involved firing alpha particles at a very thin sheet of gold and recording how these particles scattered or deflected.
Historical Background and Significance
Before Rutherford, atomic models like Thomson’s 'plum pudding' assumed positive charge spread out evenly in the atom. In 1911, Ernest Rutherford and his team performed this experiment, leading to the discovery of a compact, positively charged nucleus. This overturned the prevailing atomic theory and formed the basis of the nuclear model of the atom.
- 1904: J.J. Thomson proposes plum pudding model.
- 1911: Rutherford conducts the gold foil experiment.
- 1913: Niels Bohr improves upon Rutherford’s model with quantized orbits.
Experimental Setup and Stepwise Diagram
The setup involved a source of alpha particles, a thin gold foil, and a detecting zinc sulfide screen arranged in a circular pattern to track particle deflections. Alpha particles are emitted towards the gold foil, and their scattering angles are observed as flashes on the screen.
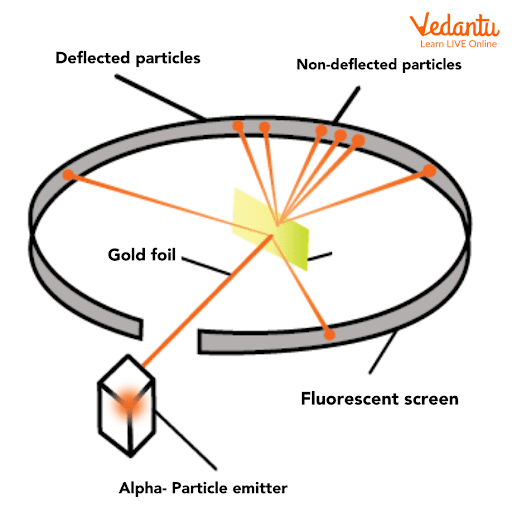
- Alpha particle source emits high-energy helium nuclei.
- Thin gold foil (few atoms thick) as the scattering target.
- Moveable detector (zinc sulfide screen) records scintillations at various angles.
Key Observations & Results of Rutherford Scattering Experiment
Three core findings redefined the atomic structure:
- Most alpha particles passed through the gold foil without significant deflection.
- Some alpha particles were deflected at large angles (>90°), though very few.
- A tiny fraction bounced nearly straight back, suggesting a very dense core inside atoms.
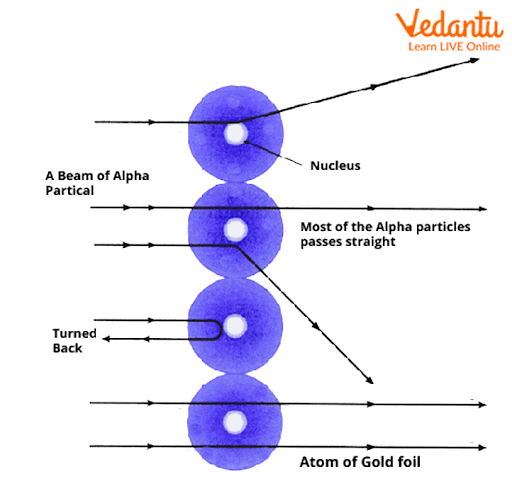
Conclusions and Nuclear Model of Atom
Based on the experiment, Rutherford’s main conclusions were:
- The atom is mostly empty space: Since most alpha particles go straight through, electrons must be tiny and far apart.
- Nucleus discovery: A minuscule, dense, positively charged nucleus exists at the center of the atom.
- Electrons orbit the nucleus like planets around the Sun.
- Almost all atomic mass is concentrated in the nucleus.
Rutherford Scattering Formula and Stepwise Derivation
The Rutherford Scattering formula gives the differential cross-section for scattering at angle θ:
| Formula | Symbol Meaning |
|---|---|
| \(\frac{d\sigma}{d\Omega} = \left(\frac{1}{4\pi\epsilon_0}\right)^2 \left(\frac{Z_1Z_2e^2}{4E}\right)^2 \csc^4\left(\frac{\theta}{2}\right)\) |
\(d\sigma/d\Omega\): differential cross section \(Z_1, Z_2\): atomic numbers of particle and target \(e\): elementary charge \(E\): kinetic energy of alpha particle \(\theta\): scattering angle \(\epsilon_0\): vacuum permittivity |
Stepwise derivation for JEE:
- Alpha particle experiences repulsive Coulomb force with nucleus: \(F = \frac{1}{4\pi\epsilon_0} \frac{Z_1Z_2e^2}{r^2}\).
- By solving equations of motion, obtain the impact parameter relation.
- Calculate the number of particles scattered per unit solid angle \(d\Omega\) at angle θ.
- Differential cross-section \(\frac{d\sigma}{d\Omega}\) shows a strong dependence on \(\csc^4(\theta/2)\), matching experimental results.
In other words, the number of particles deflected at large angles drops off sharply, which matches what was seen in the experiment and confirms the nuclear model.
Applications and Limitations of Rutherford Scattering Experiment
- Determined the approximate size of the nucleus (< 10-14 m).
- Supported atomic structure and later quantum models.
- Basis for nuclear physics studies.
- Led to deeper experiments about nuclear structure.
- Limitation: Could not explain atomic stability or electron energy levels, paving the way for Bohr’s model.
- Not applicable to scattering by light nuclei at high energies due to quantum effects.
Exam Practice: PYQs and Revision Table
You may encounter diagram-based, derivation, or conclusion MCQs on the Rutherford Scattering Experiment in JEE Main.
- State the main findings of Rutherford’s experiment and describe its setup.
- Calculate scattering angle for a given impact parameter and energy.
- Explain a consequence if most alpha particles were deflected at large angles.
- Which atomic model was disproven by Rutherford’s observations?
- Derive or state Rutherford’s scattering cross-section formula.
| Quick Revision Point | Value/Fact |
|---|---|
| Year of experiment | 1911 |
| Key discovery | Atomic nucleus exists |
| Setup | Alpha emitter, gold foil, detector |
| Observation | Most α pass through, few deflected |
| Formula | \(\frac{d\sigma}{d\Omega} \propto \csc^4(\theta/2)\) |
| Model disproven | Plum pudding |
| Limitation | No explanation for atomic stability |
For further clarity, review Rutherford's alpha scattering experiment and practice more at Atoms and Nuclei for JEE Main Physics. Vedantu provides many such summaries aligned to the JEE syllabus for smart revision.
- Explore electron discovery at Discovery of Electron, Proton, and Neutron.
- Compare with Bohr’s improvements in Bohr’s Theory of Hydrogen Atoms.
- Connect with the laws of radioactivity and properties of alpha, beta, and gamma rays.
- Review all important JEE formulas in Physics Formulas for rapid practice.
FAQs on Rutherford Scattering Experiment – Gold Foil & Atomic Structure
1. What was Rutherford's scattering experiment?
Rutherford's scattering experiment was a gold foil experiment that revealed the nuclear structure of the atom. In this experiment, alpha particles were directed at thin gold foil and their scattering patterns were observed.
Key points:
- Alpha particles were shot toward an ultrathin gold foil.
- Most passed straight through with little or no deflection.
- A small fraction were deflected at large angles.
- Very few bounced back, showing the presence of a dense, positively charged atomic nucleus.
2. What did the Rutherford experiment reveal about atomic structure?
The Rutherford experiment revealed that the atom consists of a tiny, dense, positively charged nucleus at its center, with electrons orbiting around it.
Main revelations:
- Most of the atom is empty space, as most alpha particles passed through undisturbed.
- The central nucleus contains almost all the mass and positive charge.
- Negatively charged electrons are distributed around the nucleus.
3. What was the conclusion of the Rutherford scattering experiment?
The main conclusion of the Rutherford scattering experiment was that the atom has a small, dense nucleus at its center.
Important conclusions:
- Atoms have a tiny, positively charged nucleus where most of the mass is concentrated.
- The rest of the atom is mostly empty space, allowing most alpha particles to pass through.
- Electrons revolve around the nucleus.
4. When did Rutherford perform his scattering experiment?
Rutherford's scattering experiment was performed in 1909 by Ernest Rutherford and his associates Hans Geiger and Ernest Marsden.
Key timeline:
- The experiment was conducted at the University of Manchester, UK.
- Its results were published and widely accepted by 1911.
5. What is the formula for Rutherford scattering?
The Rutherford scattering formula mathematically describes the probability of alpha particles scattering at a given angle when passing near an atomic nucleus.
The formula is:
\[ \frac{d\sigma}{d\Omega} = \left(\frac{1}{4\pi \epsilon_0}\right)^2 \frac{(Z z e^2)^2}{(4 E)^2} \csc^4 \left(\frac{\theta}{2}\right) \]
Where:
- Z: Atomic number of target nucleus (e.g., gold = 79)
- z: Charge of incident particle (for alpha, z = 2)
- e: Elementary charge
- E: Kinetic energy of alpha particle
- \epsilon_0: Permittivity of free space
- \theta: Scattering angle
6. What are the main observations from Rutherford's gold foil experiment?
The main observations of the Rutherford gold foil experiment are crucial for understanding atomic structure:
- Most alpha particles passed through the gold foil undeflected.
- A small number were deflected at large angles (greater than 90°).
- A tiny number (about 1 in 8000) bounced almost straight back.
7. Why were alpha particles chosen for the Rutherford experiment?
Alpha particles were chosen for the Rutherford experiment because:
- They are heavy and positively charged, making deflections easy to observe.
- They have enough momentum to penetrate thin gold foil.
- Their paths can reveal details about the internal structure of atoms based on how much they are deflected.
8. What are the limitations of the Rutherford scattering experiment?
While the Rutherford scattering experiment was groundbreaking, it had several limitations:
- It could not explain why electrons, which should spiral into the nucleus (as per classical physics), do not do so.
- It failed to explain the stability of atoms and their discrete spectral lines.
- It was not applicable to atoms with multiple electrons due to neglecting electron-electron interactions.
9. How did Rutherford's experiment impact later atomic models?
The Rutherford experiment fundamentally changed physics by introducing the concept of a central nucleus in the atom.
Impact on later theories:
- Overturned the plum pudding model by showing most mass and positive charge were concentrated in the nucleus.
- Laid the foundation for Bohr's model and quantum models of the atom.
- Influenced all subsequent atomic and nuclear physics research.
10. What was discovered as a result of the Rutherford scattering experiment?
Rutherford's scattering experiment discovered the atomic nucleus.
Consequences of discovery:
- Identification of a small, dense, positively charged nucleus inside the atom.
- Understanding that electrons are outside this nucleus, mostly in empty space.
- Paved the way for modern nuclear physics and chemistry.